Air Quality and Lichens - A Literature Review
By Jenifer Hutchinson, Debbie Maynard, and Linda Geiser USDA Forest Service, Pacific Northwest Region Air Resource Management Program
Updated 16-Dec-96
Table of Contents
- Introduction
- Major Pollutants
- Sulfur dioxide
- Fluoride
- Acid Rain
- Photochemical Toxin
- Metals
- Mechanisms of Accumulation
- Particulate Trapping
- Ion Exchange
- Accumulation of metals
- Physiological Responses to Pollution
- Lichens as pollution monitors
- Effects of pollution on Lichens
- Sulphur dioxide
- Effects of acid rain
- Bark pH interactions
- Soil chemistry interactions
- Other substrate interactions
- Acid fog
- Sensitivity of lichens to sulfur dioxide vs. acid rain
- Nitrogen enriched acid rain
- Lichen structure and physiology
- Effects of fluorine
- Effects of photochemical toxins
- Effects of metals
- Methods of Study
- Methods using naturally occurring lichens
- Distribution mapping
- Gradient studies
- Laboratory measurements
- Elemental Analysis
- Measuring metals accumulated by lichens
- Physiological responses
- Transplants
- Photography
- Methods using naturally occurring lichens
- Air quality monitoring studies using lichens in the Pacific Northwest
- West of the Cascade crest
- East of the Cascade crest
- Annotated bibliography
Introduction
Lichens are an integral and important component of Pacific Northwest ecosystems. They contribute to nutrient and hydrological cycles (Pike 1978, Harper and Marble 1988, Rychert and Skujins, 1974), biological diversity (McCune and Geiser 1997), and they are critical sources of forage, shelter and nesting material for many mammals, birds and invertebrates (Gerson and Seaward 1977, Richardson and Young, 1977, Hanley et al. 1989, Rochelle 1980, Maser et al. 1981,1985 1986, Gunther et al. 1983). The largest biomass of forage and nitrogen-fixing lichens occurs in late-successional and old-growth forests with clean air quality (McCune 1993, Lessica et al. 1991, Neitlich and McCune 1995). Effects of deteriorating air quality on lichens and other sensitive, ecologically critical organisms is an important management concern on national forests and other public lands of the Pacific Northwest (Peterson et al. 1992, Rosentreter 1995, FEMAT 1993, USDA-FS and USDI-BLM 1994a and 1994b). The following review describes some of the pollutants affecting lichens in the Pacific Northwest, mechanisms of pollutant accumulation by lichens, physiological responses to pollution, methods of study, and provides a summary of regional air quality studies within the Pacific Northwest utilizing lichens.
Major Pollutants
A variety of elements and chemical compounds affecting lichen growth and distribution are found in the atmosphere. Primary pollutants, including sulfur dioxide (SO2), nitrogen dioxide (NO2) and fluoride (F) compounds, remain in the same chemical form after they are emitted into the atmosphere. Secondary pollutants result from chemical reactions involving primary pollutants during atmospheric transport. This class of pollutants includes ozone (03), peroxyacetyl nitrate (PAN), and acid rain components such as sulphuric (H2SO4) and nitric (HNO3) acids. Finally, a third, mixed category of 'air toxics' includes industrial organic compounds, agricultural pesticides, trace metals, and metalloids. Information on effects of compounds in this category on lichens is not extensive (Belnap, et al. 1993). The term "air pollution", as used in this text, designates a mixture of atmospheric smoke, mineral rich dust, sulfur dioxide, nitrogen oxides, sulfur and nitrogen based acidifying compounds, fluorine, photo-oxidants such as ozone and PAN, and air toxics.
In general, the air quality in Oregon and Washington is very good compared to other areas of the United States. However, population is expected to grow from 8.1 to 9.4 million people within the next 15 years, with concomitant stresses on the region's air resources. For an extensive description of current air quality status, emission trends and deposition measurements in the Pacific Northwest region of the U.S., see Eilers et al. (1994).
Sulfur dioxide
Sulfur dioxide is a by-product of coal or fuel oil combustion, ore reduction, paper manufacture, many industrial processes, and vehicle exhaust. Natural background concentration is 0.28 to 2.8 mg/m3, but values near pollution sources can rise to as high as 200 mg/m3 or more. Most lichens cannot survive extended periods of SO2 exposure above 60 mg/m3 (Hale 1981). Sulfur emissions have declined dramatically in the last decade in the Pacific Northwest, attributable to the decline in natural emissions from Mt. St. Helens and the closure of the ASARCO copper smelter in Tacoma (Eilers et al. 1994). Total combined emissions of SO2 in 1993 for Oregon and Washington was 211,000 tons, about 15% of the national total.
Nitrogen oxide
The dominant anthropogenic sources of NO2 are fossil fuel combustion by stationary sources and vehicles. Although NOx thresholds for lichens have not been established, NOx and other nitrogenous compounds are components of acid rain and photooxidants. Effects of these pollutants on lichens are discussed in the acid rain section below.
Total combined emissions of NOx in 1993 for Oregon and Washington was 571,000 tons, about 2% of the national total. Although NOx emissions in Oregon, outside of Portland, are expected to remain low, emissions in Washington are expected to begin increasing again within the next ten years as increases in vehicle use outstrips reductions achieved through emission controls (Eilers et al. 1994).
Fluorides
Fluorides are released into the atmosphere as by-products of aluminum, zinc and phosphate ore reduction, but can also occur in fly ash from coal-burning installations. Fluoride is a locally important pollutant around smelters, brickworks, glass making factories and fertilizer plants and are also released in large amounts by volcanic eruptions (Richardson 1992). Many lichens are sensitive to this pollutant as it can concentrate in hydrated lichens to more than 200 times ambient concentrations. In general, obvious damage to lichens begins at internal concentrations between 30-70 ppm (Gilbert 1971, Perkins et al. 1980).
Acid Precipitation
Acid precipitation is rain or snow possessing a pH less than 5.6, the pH of pure water in equilibrium with atmospheric concentrations of CO2. Acid rain is produced by regional enrichment of air with SO2 and NO2 which subsequently oxidize to form sulphuric acid (H2SO4) and nitric acid (HNO3). The relative amounts of sulfuric and nitric acids vary in time and geographical location. Deposition actually takes a variety of forms, including dry, aqueous and gaseous deposits of sulfur and nitrogen based acids and oxidant pollutants, including ammonia and its derivatives (e.g. Table 1).
Table 1. Known atmospheric sulfates (Dobbins 1979).
| Name | Formula | Sources | Chemical Properties | Probable Size Class Diameter (D) |
|---|---|---|---|---|
|
Sulfuric acid |
H2SO4 |
Atmospheric oxidation of SO2; direct from manufacturing |
Strong acid; very hygroscopic (drying agent at low relative humidity |
0.1-1.0 µm |
|
Acid ammonium sulfate |
NH4HSO4 |
Oxidation of SO2 with NH3 addition |
Strong acid; hygroscopic |
0.1-1.0 µm |
|
Triammonium acid disulfate |
(NH4)3H(SO4)2 |
Oxidation of SO2 plus NH3 |
Acidic; liquefies at ~65% relative humidity |
0.1-1.0 µm |
|
Ammonium sulfate |
(NH4)2SO4 |
Oxidation of SO2 plus NH3 |
Weak acid; water-soluble; liquefies at 80% relative humidity |
0.1-1.0 µm |
|
Sodium sulfate |
Na2SO4 |
Paper pulping by kraft process |
Water-soluble; liquefies at 84% RH; relatively inert |
> 0.5 µm |
|
Calcium sulfate |
CaSO4 |
Wind-blown dust; manufacture of gypsum products |
Low solubility in water; relatively inert |
> 1 µm |
|
Magnesium sulfate |
MgSO4 |
Sea spray; paper pulping |
Very hygroscopic; relatively inert and nontoxic |
> 1 µm |
Long range transport processes distribute small particle size, acidifying pollutants to ecosystems far removed from pollution sources. Both dry and wet deposition are significant routes of acid input to forests in the western United States.
Dry deposition is an almost continuous removal of pollutants from the lower atmosphere at ground level. Acidifying compounds commonly deposited as dry deposition include sulfur dioxide (SO2), sulfuric acid (H2SO4), nitrogen dioxide (NO2) and nitric acid (HNO3). Dry deposition of both nitrate and sulfate in the western U.S. are highest in the summer months (NAPAP 1990).
The relative amount of wet deposition declines seasonally and geographically with declines in precipitation. In the Pacific Northwest, most of the annual precipitation falls in the winter. Because storms generally move in from the Pacific, pH values are usually above 5. The greatest rainfall acidity probably occurs in the summer, during light summer rains after extended drought . Acid fog and rime ice (frost) are two important types of wet deposition as the ion load is many times the load in rain or snow (NAPAP 1990).
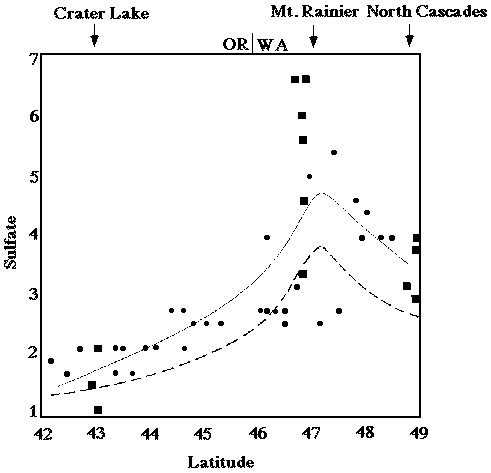
Overall annual acid input to forested ecosystems in the Pacific Northwest is relatively low compared to other parts of the nation (see figures 1 and 2; Eilers, et al., 1994). Although most western forest soils are well buffered (NAPAP 1990), wet and dry deposition can accumulate over time. The average concentrations of sulfur and nitrogen species in precipitation throughout the PNW are are not uniform. SO42- and H+ ion concentrations in snow along the Cascades increase with increasing latitude suggesting that precipitation composition in the Washington Cascades may have been degraded relative to precipitation in the Oregon Cascades (see figures 3 and 4; Eilers, et al., 1994). Poorly buffered alpine-subalpine systems are thought to be most sensitive, although the extent and magnitude of harmful effects in these systems is poorly understood.
Figure 1. Precipitation-weighted concentrations of sulfate (SO42-) ions (in mg/L) for the United States for 1992 (Source: NADP/NTN 1993). From Eilers, et al. (1994).
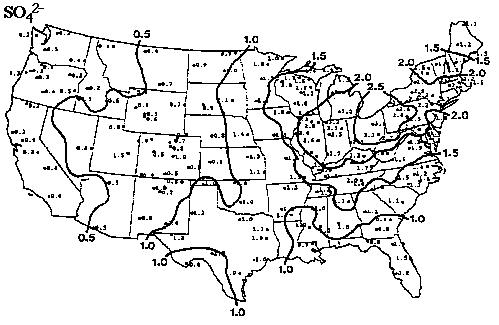
Figure 2. Precipitation-weighted concentrations of sulfate (NO3-) ions (in mg/L) for the United States for 1992 (Source: NADP/NTN 1993). From Eilers, et al. (1994).
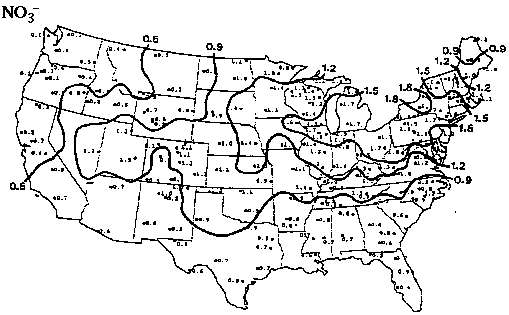
Figure 3. Concentration of hydrogen ion (µeq/L) in snow versus latitude (degrees) for 1983 from the Oregon/California border (lat. 42°) to the Washington/Canada border (lat. 49°) as reported by Laird et al. (1986). The line is a cubic spline fit of the raw data. The rectangles represent samples collected within the national parks. From Eilers, et al. (1994).
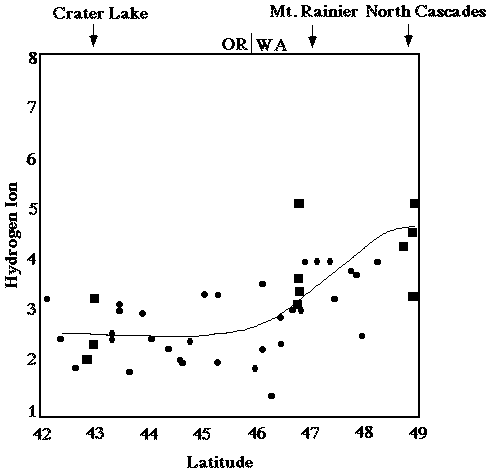
Figure 4. Concentration of sulfate (µeq/L) in snow versus latitude (degrees) for 1983 from the Oregon/California border (lat. 42°) to the Washington/Canada border (lat. 49°) as reported by Laird et al. (1986). The line is a cubic spline fit of the raw data. The dashed line represents the cubic spline fit of nonmarine sulfate. The rectangles represent samples collected within the national parks. From Eilers, et al. (1994).
Photochemical Toxins
Photochemical toxins are generally associated with automobile exhaust in cities, and form from the burning of fossil fuels. In this group are ozone and peroxyacetylnitrate (PAN). Ozone is formed by the breakdown of nitrogen dioxide (NO2) to nitric oxide (NO) and the oxygen radical (O). The breakdown reaction is catalyzed by hydrocarbons (natural and anthropogenic) under high sunlight and temperature conditions. The oxygen radical combines with molecular oxygen (02) to form the highly reactive ozone molecule (O3). PAN is formed by further reaction of ozone with breakdown products of NO2. Photochemical thresholds for lichens is a controversial topic (see discussion below). In the Pacific Northwest, exposure of vegetation to elevated ozone concentrations occurs downwind of the major metropolitan areas of Seattle, Tacoma, Vancouver, and Portland (NAPAP 1990).
Ozone concentrations higher than 80 ppb occur in the Washington Cascades during the summer months. Federal lands on the western slopes of the Cascades at higher elevations in Washington are particularly vulnerable, with peak hourly ozone concentrations ranging from 90-196 ppb. Although significant effects on forest health have not yet been demonstrated in the Pacific Northwest, based on existing does response data, these ozone concentrations could pose a threat to forest vegetation in Washington (Eilers et al. 1994).
Böhm et al (1995) described ozone regimes in and near forests of the Western United States. The 14 EPA calibrated and monitored sites in Oregon and Washington experienced small-urban, rural-remote, or remote ozone regimes:
- Small-urban ozone regimes occurred in Seattle-Tacoma, Portland, Eugene, Medford and Salem. Even though Seattle-Tacoma and Portland are major metropolitan areas, ozone formation potential is lower in the Pacific Northwest (compared to California and east of the Cascades) due to cooler average temperatures, decreased solar radiation intensity and more cloudiness. Small-urban sites are dominated by urban/small curves, with a few incidences of urban/large and medium curves (See Figure 5). Ozone maxima occur during the summer.
- Rural-remote regimes are dominated by remote curves (40-90%) with frequent inversion curves. Urban/transport, urban/small curves occur occasionally (<15%) and urban/large, urban/medium occur rarely (<5%). Pack Forest (south of Tacoma), Columbia County (NW OR), and Clackamas County (NW OR) contained rural-remote sites. Sites may be impacted by ozone precursors originating in urban areas although inversion curves also represent downward mixing of ozone from the tropospheric reservoir. Ozone maxima occur during the spring and summer.
- Remote regimes are dominated by remote curves and included Olympic National Park, Port Angeles in NW WA and Crook County in central eastern OR. High ozone concentrations typical of medium and large urban areas do not occur at these sites. Inversion and small urban curves may occur <15% of the time. Although ozone in the Olympic Peninsula has been traced to precursors form Portland and Seattle-Tacoma, overall these sites are the farthest removed from anthropogenic sources of ozone. Ozone maxima occur in the spring.
- Figure 5. Seventeen characteristic patterns of hourly ozone concentrations (ppb) identified by Böhm et al. (1995), arranged by six categories: urban/large, urban/medium, urban/small, urban/transport, inversion, and remote. Hourly means with standard deviations are plotted. The patterns are labeled using letter and number combinations: the letter refers to the shape of the curve ('A' curves have little diurnal variation; 'E' curves are characterized by larger diurnal fluctuations in ozone), and the number relates to the relative magnitude of the 24-hour mean ('1' has the lowest mean). From Bohm, McCune, and Vandetta (1995).
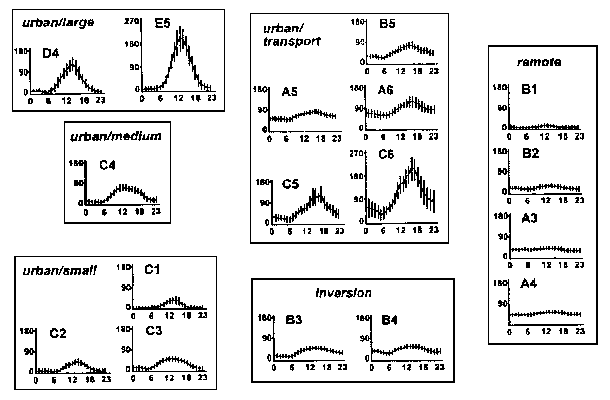
Part of the difficulty in establishing lichen sensitivity to ozone has been the difficulty in finding study areas where ozone concentrations are elevated at times of the day when lichens are metabolically active. Note that all the ozone curves in Figure 5 have maximum values between noon and 6 PM, typically the time of day when lichens are least likely to be active. Some factors which might theoretically increase the likely hood of ozone damage to lichens would be high elevation (lichens at cooler sites stay metabolically active longer into the day during the summer time because they don't dry out as fast), location of study site in an area subject to urban/transport ozone curves (broadens the length of time each day when ozone concentrations are elevated), and location of lichens in microhabitats which keep them moist longer (e.g. among mosses). Potential areas for study of lichen impacts from ozone in OR and WA would be the western WA Cascades, especially the NW slope of Mt. Rainier (see Brace 1996), and the Columbia River Gorge National Scenic Area.
Metals
Lichens are supplied with mineral nutrients and heavy metals from precipitation, throughfall, dust, and the underlying substrate from both natural and anthropogenic sources. Natural sources of metals include marine aerosols, leachates from foliage and bark, and suspended particulates derived from local and remote soils and rock. Lichens show varying sensitivity to metals, are good accumulators, and have been used to indicate deposition levels. (See sections on metal accumulation and effects). Some anthropogenic sources for specific metals are listed below.
Lead (Pb). Pb is the most important heavy metal pollutant in the atmosphere . The main source of lead in cities is leaded gas; in rural areas or isolated industrial sites, the source is any process involving coal combustion or ore reduction.
Nickel (Ni). Ni is a by-product of coal combustion, industrial processes, and automobile exhaust. Background levels in the U.S. range from 3 to 9 ppm (Hale 1981).
Mercury (Hg). Hg is a minor component of air pollution in the Pacific Northwest from the burning of fossil fuels.
Chromium (Cr). Cr is a component of stack fly-ash and presumably travels long distances in the atmosphere; it's presence can be correlated with industrial centers.
Zinc (Zn). Zn is emitted from zinc smelters, and is also produced in automobile exhaust (Hale 1981).
Titanium (Ti) and Vanadium (V). Ti and V are considered signature elements of petroleum combustion pollution; enhanced levels of these elements strongly indicate anthropogenic sources (Wetmore 1987b).
Mechanisms of Accumulation
Lichens accumulate substances from their environment by a variety of mechanisms, including particulate trapping, ion exchange, extracellular electrolyte sorption, hydrolysis, and intracellular uptake (Nieboer et al.1978).
Particulate Trapping
Particles of various elements, such as iron, titanium, aluminum, chromium, and uranium can become embedded in the lichen thallus in the algal and/or fungal layer under moist or dry conditions (Nieboer et al. 1978, Gough & Erdman 1977). Morphological characteristics that contribute to particulate trapping include the microtopography of the lichen surface (Richardson & Nieboer, 1980), and a large surface area-to-volume ratio of the lichen, which can result from a thin thallus, branching, or projections from the thallus such as isidia and phyllocladia (Tomassini et al. 1976, Tomassini 1976).
Ion Exchange
Ion exchange requires that the lichen must be moist, so that the elements are in ionic form. Uptake of metal ions by ion exchange is passive and rapid and obeys the principals of mass and charge balance. Extracellular binding sites, such as at cell walls, are most likely involved.
Lichen species vary in their capacity for ion-uptake, as well as the particulate trapping efficiency of their surface morphology. This underlines the importance of correct identification of lichen samples used for monitoring purposes and demonstrates the desirability of collecting the same species for analysis from all sampling sites.
Accumulation of metals
The action of rain, surface water, and passive upward diffusion from the substrate likely bring dissolved minerals in contact with lichen thalli (Nieboer et al. 1978, Nieboer & Richardson 1981). The amount of each type of metal ion that can be accumulated by a lichen is dependent upon the uptake characteristics of that particular species and the amount and availability of metal ions in the surrounding environment. Extracellular uptake of metal ions is essentially a passive process of ion exchange determined by the character of the ligands in the fungal cell walls. Intracellular uptake is limited by the nature of the metal ion, cell membrane permeability, and the concentration of extracellular ligands with affinity for cations (Tyler 1989).
Lead. Lichens are very efficient accumulators of Pb, through aerosols, particulate metal fallout, or acid rain. Pb is bound to insoluble anionic sites, accumulated extracellularly, and concentrated in the medulla. Once bound, Pb is not easily removed by rain or wind. The toxic effects of Pb on lichens is minimal. Some species can accumulate up to about 2000 ppm, after which concentrations do not increase, indicating a degree of physiological turnover. Surveys of Pb content have proved useful in establishing background (preindustrial) values which can be referenced to present levels (Lawrey & Hale 1981).
Nickel. Ni is taken up by algal cells in lichens.
Mercury. Hg is readily accumulated by lichens. Background values were measured at approx. 0.223 ppm, while specimens 8 km from a chlor-alkali plant contained 0.53 ppm (Lodenius, 1981).
Chromium. Schutte (1977) reported Cr accumulation in lichens of Ohio increased two to ten-fold over turn of the century concentrations.
Copper. Nieboer et al. (1979) documented two phases of Cu uptake in Umbillicaria muhlenbergii. In the first phase copper ions bond to receptor sites on algal cells. In the second phase, copper ions bind to the fungal cells, as evidenced by K+ efflux.
Zinc. Zn is found in fairly large concentrations (200-600 ppm) in lichens near zinc smelters.
Physiological Responses to Pollution
Lichens as pollution monitors
Lichens and bryophytes possess a number of characteristics that make them suitable biomonitors for air pollution. Many lichen species have large geographical ranges, allowing study of pollution gradients over long distances. Lichen morphology does not vary with the seasons, and accumulation of pollutants can occur throughout the year. Lichens are usually very long lived.
Lichens are also suitable biomonitors due to their sensitivity to pollution. Several factors contribute to this sensitivity. Water and gas are exchanged over the entire lichen thallus. Because they lack roots, lichens do not have access to soil nutrient pools and must depend on deposition, water seeping over substrate surfaces, atmospheric and other comparatively dilute sources of nutrients. Thus, their tissue content largely reflects atmospheric sources of nutrients and contaminants. Lichens also lack the protective tissues or cell types necessary to maintain a constant internal water content. Many lichens pass through multiple wetting and drying cycles during a day. When hydrated, nutrients and contaminants are absorbed over the entire surface of the lichen. During dehydration, nutrients and many contaminants become greatly concentrated in the lichen tissues, i.e., absorbed to cell walls, cloistered inside organelles or crystallized between cells. Different contaminants have different release rates depending on the way they are stored. Nutrients tend to be more easily remobilized than metal contaminants. During heavy rains, nutrients and pollutants are gradually leached. A dynamic equilibrium thus exists between atmospheric nutrient/pollutant accumulation and loss, which makes lichen analysis a sensitive tool for the detection of changes in air quality. Cryptogams have consistently shown higher metal levels than higher plants (Rühling& Tyler 1968, Huckaby 1993, LeBlanc et al. 1974, Hocking et al. 1978, Solberg & Selmer-Olsen 1978, Arafat & Glooschenko 1982). In one study, lichen metal levels reflected the prevailing wind direction to a greater extent than did pine needles (Laaksovirta & Olkkonen 1977, 1979).
Different morphological types of lichens exhibit differing levels of sensitivity to pollution as a result of combined factors that are not well understood. In general sensitivity increases in the following series: crustose < foliose < fructicose.
Effects of Pollution on Lichens
A myriad of pollution effects on lichens have been described. At the level of the whole plant, investigators have described decreases in thallus size and fertility (DeWit 1983, Kauppi 1983, Sigal & Nash 1983), bleaching and convolution of the thallus (Sigal & Nash 1983), restriction of lichen occurrence to the base of vegetation (Sigal & Nash 1983, Neel 1988), and mortality of sensitive species (DeWit 1983, Denison & Carpenter 1973). Microscopic and molecular effects described include reduction in the number of algal cells in the thallus (Holopainen 1984), ultrastructural changes of the thallus (Anderson & St. Clair 1983, Hale 1983, Holopainen 1984, Pearson 1985), degradation of photosynthetic pigments (Kauppi 1980, Garty et al. 1993), altered photosynthesis and respiration rates (Sanz et al. 1992, Rosentreter & Ahmadjian 1977), and elevation in the content of heavy metals in the thallus (Addison & Puckett 1980, Carlberg et al. 1983, Gailey & Lloyd 1986 a, 1986b and 1986c; Gough & Erdman 1977, Lawry 1986). Huebert et al. (1985) concluded that peak exposures may be of primary importance in determining the survival of lichens in industrial areas.
Sulphur dioxide
SO2 is considered to be the primary factor causing the death of lichens in most urban and industrial areas, with fruticose lichens being more susceptible to SO2 than many foliose and crustose species (Seaward 1987). The observed effects of SO2 include decreases in respiration and photosynthesis, increases in membrane permeability, increases in K+ influx and ions lost, and ultrastructural changes (Belnap et al. 1993). Damage to the algal component of the thallus is evidenced by its discoloration. The entire thallus dies soon after algal cells are damaged (Hale 1981, Wetmore 1985). Low pH increases the toxicity of SO2 action (Farmer et al. 1992)). Nieboer et al. (1979) determined that copper also enhances the effects of SO2.
There are three general mechanisms by which SO2 exerts it's toxic effects upon the lichen thallus. At low pollution levels, enzyme systems within the thallus may become activated. This can be observed as increases in glucose-6-phosphate dehydrogenase activity, or an increase in the levels of glutathione and total protein SH. Heightened enzyme activity can be used as a bioindicator for the non-visible injury metabolism and detoxification of absorbed SO2 (Rabe and Kreeb 1980, Miszalski & Zeigler, 1979, Grill et al. 1980). At higher levels, SO2 deactivates enzymes by chemical modification (sulfitolysis) leading to reduced metabolic activity and loss of membrane integrity (Zeigler 1975 and 1977, Nieboer et al. 1976). It also binds to the central metal atoms of enzymes, adversely affecting membrane function and cell osmolality (Malhotra and Khan 1980, Gunnison et al. 1981, Mansfield and Freer-Smith). In addition, SO2 competitively inhibits carbonate (HCO3) and phosphate (H2PO4) interactions with enzymes (Alscher-Herman 1982).
Table 2. General Mechanisms of SO2 toxicity
| Type of reaction | Observed/expected response or injury | References |
|---|---|---|
|
Reduced metabolic activity; loss of membrane integrity, membrane function and cell osmolality, (competition with HCO3-, H2PO4-) |
Zeigler 1975, 1977, Nieboer et al. 1976, Malhotra and Khan 1980,Gunnison et al. 1981, Mansfield & Freer-Smith 1981, Alscher-Herman 1982 |
|
Use of increases in enzyme activity as a bioindicator for non-visible injury and detoxification of metabolism absorbed SO2 |
1979, 19 Rabe & Kreeb, 80, Miszalski & Zeigler, 1979, Grill et al. 1980 |
|
Modification of metabolic precursors and products; interference with electron flow in photosynthetic and respiratory electron-transport chains. |
Puckett et al. 1973; Ziegler, 1975; 1977; Gunnison, 1981.Nieboer et al, 1976; Shapiro, Petering & Shih, 1975; (108) |
Effects of Acid Rain
Natural and anthropogenic variations of substrate and water acidity are a major factor governing the composition and health of bryophyte and lichen communities (Farmer, et al. 1992). At the level of the individual thallus, acidity affects nitrogen fixation, photosynthesis, growth, and cellular ultrastructure (Belnap et al. 1993). Besides these direct effects on physiological processes, environmental acidity can affect cryptogamic communities indirectly by altering substrate chemistry, thereby affecting species diversity and composition at the community level.
Bark pH interactions
Ecological studies of epiphyte communities have shown that, even in unpolluted areas, natural variations in bark chemistry have a major impact on which species are present ( Farmer, et al. 1992). For example, Hale (1955) found that species of Physcia, Xanthoria, and Candelaria were more common on trees with bark pH values of 5.3 to 6.6. Many factors can influence bark pH. Tree species differ in this characteristic; for instance, conifers generally have lower bark pH than angiosperms. Other influential factors affecting the number and type of epiphytes present include the buffering capacity of the bark, dust accumulation, and the age of tree. Individuals of a single tree species can have markedly different bark chemistries and epiphyte communities (Farmer et al. 1992). For example, Gilbert (1970) showed that the pH of Fraxinus could vary on a single tree from 5.7 along a nutrient streak to 2.2 on the underside of a branch.
Gough (1975) speculated that continuous peeling off of bark scales may be one reason for little epiphytic cover on Pinus ponderosa in Colorado. He found that P. ponderosa bark loses water faster than the bark of aspens and Pseudotsuga menziesii, Abies lasiocarpa, Picea engelmanii, and Pinus contorta. Distribution of lichens on trees may also be related to pH. The pH of the outermost newly-exposed orange bark of trees over 4 dm in diameter tended to be significantly lower (pH 3.5-4.27) than that of bark in furrows (pH 3.71-4.66) of the same trees and bark of smaller trees (pH 3.76-4.84). The pH of bark on twigs averaged about 4.78, significantly higher than bark on trunks; lichen cover on branches and twigs was generally more continuous on than on tree trunks.
The presence of pollutants also affects bark pH. Gilbert (1965) studied the pH of the bark of Fraxinus excelsior and Acer pseudoplatanus in and around Newcastle. While the average pH value of F. excelsior was 4.2 both at 31 km from Newcastle and in the city itself, the bark pH of A. pseudoplatanus dropped from 4.8 at 31 km from Newcastle to 3.5 in Newcastle. Robinia psuedoacacia trees growing in the vicinity of a pulp mill (Hoffman 1974) were found to have bark pH values of 6.4 to 5.2, with the lower values generally corresponding to the trees closest to the mill.
British researchers have noted that bark pH is a critical factor in the establishment of acidophilous species of the Parmelia laevigatae community, normally associated with high elevation woodlands. With continuing acidification these species can replace the lower elevation Lobaria community. Acid rain can reduce pH of both tree bark and lichen thalli (Farmer et al., 1992).
Soil chemistry interactions
Variations in soil chemistry may be important. For example, Norwegian oaks with Lobarion species grew mainly on nutrient rich soils. Variations in bark chemistry may be affected by variations in soil cation availability. The relationship between soil and bark chemistry is further complicated by the ability of trees with acidic stemflow to acidify the surrounding soil, as demonstrated for Fagus sylvatica in Sweden (Farmer et al, 1992).
Bark and soil acidification is caused by the total H+ ion input to the system. The critical H+ ion deposition load for epiphytes is the highest load that will cause a change in surface bark chemistry, particularly of base saturation, that can be countered by natural chemical and weathering processes (Farmer et al. 1992). For epiphytes, extreme events are evened out by the buffering capacity of bark, so that the best predictions of epiphyte response are probably made on annual averages of pollutant loading. Lichens may respond to short episodic pollution events within a local area that may be undetected by conventional analysis.
Other substrate interactions
Evidence from field observations, intensive studies of the environment at contrasting sites, and field and lab experiments (Farmer et al. 1992) show that acid rain can affect lichens both directly and indirectly by the acidification of substrata. Seaward (1987) writes, "Regional pollution over wide areas has reduced lichen diversity, and has favored the expansion of a relatively small number of species formerly having narrower ecological requirements and/or more restricted distributions such as Buellia punctata, Lecanora conizaeoides, Lecanora muralis, Parmelia incurva, Parmeliopsis ambigua, Phaeophyscia orbicularis, Scolisciosporum chlorococcum, Xanthoria candelaris, and Xanthoria elegans." Pollution most greatly affects lichen species sensitive to high concentrations of H+ and Al3+. In addition, when pollution occurs in poorly buffered environments, species such as Lobaria which normally thrive on mildly acidic to neutral substrate, will also be negatively affected (Farmer et al. 1992).
Acid fog
Fog, which often increases with increasing elevation, contains higher levels of dissolved ions, including H+, than precipitation rain or snow. Although the effects of occult deposition from fog on epiphyte communities have not yet been critically examined, acid fog has been implicated in changes to terricolous cryptogamic floras (Wolseley & James 1992). Desert lichens, which obtain most of their water from fog and dew, are particularly vulnerable to air quality and weather pattern changes (Follman 1995, Nash 1996).
Sensitivity of lichens to sulfur dioxide vs. acid rain
Species tolerant to acid rain need not be the same as those tolerant of SO2. Usnea species intolerant of SO2 grew on bark of low pH, while Parmelia sulcata was tolerant of SO2, but not of acid rain (Gilbert 1986).
Nitrogen enriched acid rain
The nitrogenous component of acid rain can produce a fertilizer effect on lichens and cause floristic changes. Søchting (1990) surveyed the tissue nitrogen content of reindeer lichens in Denmark. In unpolluted areas, tissue levels were 0.26-0.49 % while in areas of wet deposited acidity, values were 0.70- 0.73 % and visible injury could be found. However, the problem of defining critical loads for nitrogen has not been resolved. Presently, this is determined by the biological response of the system, and is very hard to quantify, compared to H+, which is measured by buffering capacity (Famer et al. 1992).
Lichen structure and physiology
The effects of acid rain on lichen structure and physiology include a decrease in nitrogen fixation, photosynthesis, and growth, and changes in cellular ultrastructure (Belnap et al. 1993). Hutchinson et al. (1986) treated Cladina rangiferina and Cladina stellaris with artificial acid rain for more than five years. They found reduced photosynthesis and chlorophyll levels, and decreased dry weight and podetial height of thalli of both species receiving pH 2.5. No effects were observed at pH 3 and above. Nitric acid applied alone at pH 2.8 caused dry weight increases of 62% over controls in C. rangiferina with an increase in tissue nitrogen levels. Sulphuric acid applied alone caused a significant reduction in dry weight.
Effects of Fluorine
The ability of lichens to accumulate F is a function of relative humidity, which determines the moisture conditions of the thallus. In general, obvious damage to lichens begins at levels of 50-70 ppm (Gilbert 1971). The effects of fluorine include a decrease in respiration and photosynthesis, an increase in membrane and thallus permeability with a concomitant loss of ions, and changes in cellular ultrastructure (Belnap et al. 1993, LeBlanc et al. 1971).
Lichens exposed to ambient F at 4 mg F/m3 accumulated F within their thalli, and eventually surpassed the critical concentration of 30-80 ppm (Perkins et al., 1980). Both in the field and in the lab, whenever the level of F within the thallus exceeded 80 ppm, chlorosis was observed. Subsequently, all the photosynthetic pigments were degraded and the lichen thalli disintegrated. In the field, wherever chlorotic transplants were found, high levels of F on lime filter papers were also found, suggesting that ambient F was the cause of the lichen injury. The lichens used in this study were Cladonia cristellata, C. polycarpoides and Parmelia plittii (Nash 1971).
Perkins et al. (1980) showed that F accumulation by corticolous lichens was negatively correlated with distance from an aluminum reduction plant and that lichen damage and reduced abundance was due to F. SO2 emitted in combination with HF from a mix of industries in Whatcom County, Washington, was associated with a serious depletion of the lichen flora, even though emission levels were within acceptable limits based on human health standards set by the US Environmental Protection Agency (Taylor & Bell 1983).
Effects of photochemical toxins
Ozone, PAN and nitrogen oxides are toxic to lichens in sufficient concentrations. Ozone effects noted by researches have included decreases in photosynthesis, reduction in the geographical distribution of a species, and morphological and ultrastructural changes. PAN can also affect photosynthesis and cause ultrastuctural changes, while NOx effects are expressed in the loss of chlorophyll pigment (Belnap et al. 1993). In higher plants, oxidant air pollution is thought to disrupt normal pathways of energy by altering cell membranes; no comparable data for lichens exists, and experiments to determine the effects of ozone on lichens have yielded contradictory results.
Field studies in the Los Angeles basin (Neel 1988, Sigal & Taylor 1979, Sigal & Nash 1983) show a broad correlation between lichen loss and increases in phototoxins in the past 80 years; however, phototoxins are difficult to research, as they are produced over a large area in low concentrations and have at most long-term or chronic effects on lichens (Hale 1981). Ozone appears to have little visible effect on roadside lichens (Lawry & Hale 1979). Lichen communities in the Indianapolis area were insensitive to differences in ozone levels. This could be due to an intrinsic species insensitivity to ozone, or from the fairly narrow ozone dose range observed. Over the three year study period the means of ozone deposition differed by 12 mg/m3 across sites, whereas means for SO2 differed by 17 mg/m3 (McCune 1988). Photochemical toxins may have synergistic effects when combined with other pollutants, especially under low pH conditions but this synergy has not been conclusively documented (Farmer et al. 1992).
Ruoss and Vonarburg (1995) studied lichen metabolic activity in relation to ozone concentrations at three elevations in central Switzerland. They also fumigated lichens at 300 ppm for 34 days. While the fumigated lichens showed damage (bleaching and discoloration), the specimens from the field experiment were not differentially affected by ozone. The authors found that ozone levels never exceeded 120 ppm when relative humidity was high enough to sustain lichen metabolism and, therefore, lichens were not susceptible to ozone during periods of high ozone concentrations. While lichen diversity and abundance increased with elevation the highest ozone levels occurred at the highest elevations. The authors felt lichen diversity and abundance was primarily impacted winter pollution, i.e. acidic deposition, which decreased with elevation.
Effects of metals
Metal ions can be classified into three groups. Class A ions: K+, Ca2+, and Sr2+ are characterized by a strong preference for O2-containing binding sites and are not toxic. Ions in the B class: Ag+, Hg+, Cu+, tend to bind with N and S-containing molecules, and are extremely toxic to lichens even at low levels. Borderline class ions: Zn2+, Ni2+, Cu2+, Pb2+, are intermediate to Class A and B ions. Borderline ions, especially those with class B properties, e.g. Pb2+, Cu2+, may be both detrimental by themselves and in combination with sulphur dioxide (Nieboer & Richardson 1981).
The toxicity of metal ions is the result of three main mechanisms: the blocking of essential biological functional groups, the displacement of essential metal ions, and the modification of the active conformation of biomolecules. In studies of specific ions, Nieboer et al. (1979) found that Pb2+ also affects cell wall permeability and causes K+ efflux, which is correlated with a decrease in carbon fixation.
Limited field data is available concerning threshold concentration of metals in lichens. Tolerance mechanisms mainly involve immobilization of the toxic metal ions in biologically inactive forms. Tolerance to metals may be phenotypically acquired, but sensitivity of lichens to elevated tissue concentrations of metals varies greatly between species, populations, and elements (Tyler 1989).
Methods of Study
Lichens have been used to assess deposition and air quality in hundreds of studies worldwide. Reviews of the literature on this topic include Richardson (1992), Stolte et al. (1993), Nash & Wirth (1988), Nash (1989) or see the bibliography to this document. The Lichenologist publishes an ongoing series "Literature on air pollution and lichens". Below we provide examples of different approaches.
Methods using naturally occurring lichens
Distribution mapping
Distribution mapping is the classic field method for studying lichens. It was also the first method used to indicate air quality with lichens (Showman 1988). Different kinds of maps can record the total number of species per site (richness), the percentage of the quadrants in which particular species can be found (frequency), presence or absence of indicator species, and the estimated or measured cover.
Mapping lends itself well to longitudinal studies that document temporal change. Using this method, Sigal and Nash (1983) compared lichen communities in the San Bernadino and San Gabriel Mountains of California in 1983 to epiphytic macrolichens on conifers reported by Hasse in the same area in 1913. A number of species were no longer found: Nodobryoria abbreviata, Bryoria fremontii, Nodobryoria oregana, Alectoria sarmentosa, Calicium viride, Cetraria canadensis, Platismatia glauca, Evernia prunastri. Other species, such as Cetraria merrilli, Parmelia quercina, Ramalina farinacea, and Cladonia spp., were found in only trace amounts. Species that were present included Hypogymnia enteromorpha, Melanelia elegantula, Melanelia subolivacea, and Letharia vulpina.
One disadvantage of presence/absence distribution mapping is that a species must disappear before an effect can be registered. To address this shortcoming, De Sloover & Leblanc (1968) developed the Index of Atmospheric Purity (IAP). At each site, this method assigns numerical values to characteristics such as frequency and cover. Then, using a mathematical formula, it reduces these values to a single IAP value for each site. The IAP values are then mapped. The primary limitation of IAP values is that they are not comparable from one geographic region to another. Even in the absence of pollution, climatic differences between regions can produce changes in the lichen communities that underlie the IAP values (see Hoffman 1974).
Gradient studies
Gradient studies correlate available pollution exposure data with observations of visible injury, species richness and species abundance.
Linear regression analysis is used to investigate the interrelationship between variables, and to predict one variable when the other is known. Taylor and Bell (1983) used regression to show that, at the 95% confidence level, there was a functional relationship between distance from a smelter in Tacoma, Washington and total lichen cover, with an increase in cover with increasing distance from the smelter. Compared to data collected in 1976, the SO2 emissions from the smelter and the mean concentrations of sulfur found in leaves of alder trees at the study sites had both decreased. This suggested that the correlation between lichen growth and distance from the smelter was more or less established during earlier periods of greater ambient SO2 levels.
Multivariate analysis of epiphytic macrolichen survey data was used to assess air quality in the southeastern United States (McCune et al. 1994). Two major gradients were revealed. The first gradient corresponded to a climatic gradient from the coast to the Appalachian mountains. The second gradient corresponded primarily to air quality, with pollution-tolerant species and lower species richness at one end of the gradient and pollution-sensitive species and high species richness at the other end of the gradient. The gradient model was then used to calculate air pollution impact scores for sites to answer questions regarding the spatial pattern and trends of air quality effects on forest resources.
Laboratory Measurements
Laboratory methods used to study the chemical, morphological and physiological responses of lichens to pollution include elemental analysis, physiological measurements, transplant studies, fumigation studies and photography.
Elemental Analysis
Methods of elemental analysis include atomic absorption spectrophotometry, neutron activation analysis, and X-ray fluorescence spectrometry (Nieboer & Richardson 1981). Commonly analyzed elements include: S, N, Zn, F, Pb, Ni, Cu, Fe, Mg, Mn, Cr, Cd, Zn, Ca, and several rare earth elements. A large body of information is developing on elemental content of lichens, both in natural states and under pollution stress (Nash 1996). Hale (1981) recommended that every baseline study should include elemental analysis of lichen indicator species for future reference.
Using the gradient method, element concentrations within the lichen are usually observed to increase as the distance to the suspected source decreases. Gough and Erdman (1977) used linear regression to evaluate the relationship between distance from a coal fired power plant and metal levels in Xanthoparmelia chlorochroa. However, as Puckett (1988) point out, concentrations of many elements will not reach zero at large distances from pollution sources because they have essential nutritional roles or are normal components of the lichen when growing in its natural environment. When, both natural and anthropogenic sources contribute to the metals measured, identifying the relative contribution from each source becomes a complex task. Puckett (1988) reported a method of calculating "enrichment factors" to compare the concentration of metal within a plant with potential sources in the environment. The equation is
EF = x/reference element in lichen / x/reference element in crustal rockStable sulfur isotope ratios in combination with multi-element analysis of lichens were used to examine the influence of emissions from two coal-fired power plants i n the Yampa Valley on pollutant deposition in the Mt. Zirkel Wilderness of northern Colorado (Jackson et al., 1996). Coal-fired power plants typically emit SO2 with a stable isotope ratio 34S/32S characteristic of the coal combusted. Stable S isotope ratios in Usnea were significantly heavier (more positive) in the wilderness and nearby sites compared to more distant regional sites and corresponded well with sulfur isotope ratio found in snow in the same area and average isotope ratios in coal used by the power plants. Sulfur isotope studies are most easily interpreted when the point source of concern is the predominant source of sulfur in the study area. Xanthoparmelia cumberlandia from the Mt. Zirkel area were also elevated in sulfur, nitrogen, potassium, sodium and phosphorus compared to more distant regional sites (>100 km).
Determining relevant elements is an important part of any multi-element study. In Switzerland, Herzig et al. (1989 & 1990) used multivariate analysis to compare element concentrations in Hypogymnia physodes to total air pollution as assessed by lichen communities using the IAP index and an instrumented monitoring network. Four groups were discerned.
- Group 1: Ca. Calcium was the only element which increased with improving air quality.
- Group 2: Pb, Fe, Cu, Cr, S, Zn and P. Concentrations of these elements decreased in distinct curvilinear gradients with decreasing total air pollution. For example, the concentration of Pb was reduced six-fold in the "very low pollution" compared to the "critical air pollution" zone. These elements were strongly correlated with annual average atmospheric deposition measurements detected by the instrument network.
- Group 3: Li, Cd, Co. Concentrations of these elements were lower in the "very low pollution" zone than in the "critical air pollution", but the gradients were not strictly curvilinear.
- Group 4: Al, B, K, Mg, Mo, Na, Ni, Sn, Cl and organic S. These elements showed no clear gradients to the total air pollution..
Other cryptogams, especially mosses, have also been successfully used as pollution monitors (Winner 1988). Between 1989 and 1992, twenty-one European countries participated in an unprecedented effort to map atmospheric toxic metal deposition based on moss analysis (Rühling 1994). Colored contour maps of the continent for As, Cd, Cr, Cu, Fe, Pb, Ni, Vd, Zn deposition were made from metal analyses of Hylocomium splendens, Pleurozium schreberi and some secondary species. A strong linear correlation between average annual atmospheric deposition and concentration by moss was observed for As, Cd, Cr, V, and Zn. which could be used to calculate atmospheric deposition. In northern Europe, metal concentrations generally increased from north to south with local toxic metal enhancements superimposed on the regional background pattern at the large smelting region of Russia in the western Kola Peninsula. In central Europe, major enhancements were detected around the industrial regions of the Ruhr area in Germany, western Czech Republic and adjacent eastern Germany, and southern Poland.
Physiological responses
The physiological responses of lichens to pollution can be observed and measured directly. Plasmolysis of cells of the algal component of the thallus can be measured microscopically. Respiration rates have also been measured. Von Arb et al. (1990), observed changes in the fine structure of chloroplasts. Chlorophyll content, as a measure of SO2 damage, was determined spectroscopically or by microfluorometry by Beltman et al. (1980), Kauppi (1980), Kauppi and Mikkonen (1980) and Gries et al (1995).
Transplants
Lichen transplants are used to assess air quality in areas where lichens are absent or sparse. Richardson (1992) reviews the use of transplants to assess air quality in urban environments and to monitor contaminants in air and water. Pearson (1993) discusses advantages and limitations of transplant methods. Ideally, healthy lichens are transferred from an area where they occur naturally to an area to a test area. Changes in physiology or element accumulation as a result of exposure to pollution are then studied. Physiological studies are most likely to be successful when using species well within their normal range of adaptation both at the source and at the test area.
Transplant techniques for common lichens in Pacific Northwest environments west of the Cascade Range crest are discussed by Shirazi et al. (1996) McCune et al. (1996) and Sillett (1994). Hypogymnia physodes, a fairly pollution tolerant lichen widely distributed in the Pacific Northwest, has been one of the most frequently transplanted lichens world-wide (Brodo 1966, Holopainen 1984, Farkas et al. 1985, Gailey et al. 1985, Vestergarrd et al, 1986, Søchting 1995).
Fumigation
Fumigation studies involve the controlled introduction of pollutants to lichens in either a closed chamber that allows for air movement, or in a chamber-free field environment. The purpose of this kind of study is to develop quantitative relationships between concentrations of air pollutants and various anatomical and physiological lichen responses. The anticipated outcome of air pollution fumigation experiments is a clear exhibition of dose-response relationships. Thus, fumigations for a long period of time at a low pollutant level would be expected to have the same effect as a shorter fumigation at a higher concentration where the dose, measured as concentration multiplied by time, is equal for each fumigation (Sanz et al. 1992) . The most commonly measured responses are physiological, such as photosynthesis, respiration, nitrogenase activity in blue green phycobionts, K+ efflux and/or total electrolyte leakage from the thallus, and pigment status.
A problem inherent in fumigation studies is that different portions of the thallus differ in their sensitivity and physiological response to pollutant exposure. Factors such as the hydration level and physiological status of the thallus, as well as the differential reactions of the algal component vs. the fungal component, can affect its sensitivity to exposure. In addition, the concentration and duration of exposure, and the environmental conditions under which exposure occurs, can also affect sensitivity (Fields, 1988).
A strength and limitation of fumigation studies has been the tendency of researchers to expose their experimental material to only a single pollutant under a given set of conditions. Air pollution involves multiple contaminants (Belnap et al. 1993), and it is important to develop methods to test for the synergistic effects of pollutants found in the field (Fields, 1988). Farmer et al. (1992) also stressed the need for long term fumigation studies employing realistic pollutant concentrations, as the way to demonstrate conclusively whether the levels of pollutants measured in the field were actually damaging to cryptogamic flora.
Researchers evaluating fumigation experiments also need to remain mindful of the ecological conditions under which the studies are carried out (Farmer et al. 1992). For example, water relations play a significant role in the uptake of SO2 by lichens (Grace et al. 1985). Dry lichens absorb far less SO2 than moist ones and are resistant to SO2 fumigation (Coxson 1988). The low humidities used in some studies (Showman 1972, Beekley & Hoffman 1981), could have completely dried the thalli. Thus, the finding that responses in some studies appear to be independent of exposure time is not surprising (Fields & St. Clair 1984; Huebert et al. 1985). With poikilohydric plants such as lichens, it is difficult to maintain the water status and hence metabolic activity of a sample for more than a few hours. Complex laboratory equipment is necessary to successfully conduct fumigation studies.
The relationship between seasonal environmental conditions and pollution is an important adjunct to the findings of SO2 fumigation studies. For instance, NO2 reaches high levels in the winter when lichens and bryophytes are more likely to be wet and physiologically active. O3 reaches its peaks in the summer. However O3, unlike SO2 and NO2 is not readily soluble in water and may damage cell membranes even in a dry state (Farmer et al. 1992). The time of year also affects lichen net assimilation rates; for example the net assimilation rates for Peltigera praetextata and Parmelia sulcata in June increased five times over the net assimilation rates recorded from October to March (Kershaw 1972).
Even though the various gaseous pollutants have different physiochemical properties, they affect the physiological systems of lichens in similar ways (Fields 1988). In general, greater concentrations of, and longer intervals of exposure to SO2 result in reduced photosynthesis and reduced respiration, although at low levels and short exposures SO2 can enhance 14C fixation. SO2 also produces membrane damage leading to K+ efflux and total electrolyte leakage in a time dependent manner, with massive K+ release occurring at increasingly lower SO2 levels as exposure time is increased (Fields 1988). Oxidants, O3 and PAN reduce photosynthesis. The order of sensitivity of lichen physiological processes to fumigation appears to be:
N2 fixation > K+ efflux/total electrolyte leakage> photosynthesis and respiration > pigment status.
Table 4. The physiological responses of lichens after exposure to fumigated pollutants (Farmer et al., 1992, Fields 1988).
| Response | Pollutant |
|---|---|
|
Reduced photosynthesis |
SO2, NaHSO3, Na2S2O5, O3, PAN. |
|
Reduced respiration |
SO2 |
|
Decreased chlorophyll content |
SO2, NO2, HF |
|
Increased electrolyte leakage, loss of K |
SO2, HF? |
Reduced nitrogen fixation |
SO2, H2SO4 > loss w/< pH > exposure, NaHSO3, NaF |
Photography
Some researchers have used photography as a way of monitoring air quality (e.g. Hale 1981). Marked sites can be rephotographed over time to compare growth rates and condition of individual lichen colonies. Care must be taken to distinguish between air pollution and local habitat stresses.
Air quality monitoring studies using lichens in the Pacific Northwest
Air pollution studies using lichens in the Pacific Northwest west of the Cascade ranges are gradually accumulating in number. Rhoades (1988) examined lichen communities and chemistry in Olympic National Park. (Gough et al. 1988) established baselines in Redwood National Park in northern California. Taylor and Bell (1983), and Johnson (1979) examined lichens in the Seattle-Tacoma area. Moser et al. (1983) investigated the impact of emissions from Mt. St. Helens on two lichen species of South-Central Washington. Geiser et al. (1994) working in southeastern Alaska, established element concentration baselines for Cladina rangiferina, Lobaria oregana, Hypogymnia enteromorpha, and Alectoria sarmentosa, lichens widespread in the Pacific Northwest. Rhoades (1996) reviewed the literature of tissue chemistry for lichens occurring in the Pacific Northwest. McCune and Geiser (1997) proposed sensitivity ratings for ninety-three lichens using field based, lichen community studies in western Washington and Oregon. Dennison and Carpenter (1973) documented air pollution in the Willamette Valley of Oregon. The USDA Forest Service and USDI National Park Service established air quality monitoring methods for using lichens (Stolte et al. 1993) and the EPA/USFS sponsored national FHM program has protocols (McCune 1994 ) currently used in adapted form (Geiser 1995) within the Pacific Northwest region of the USDA Forest Service for lichen monitoring. This program has accumulated a large database of lichen survey and chemistry information on a 3.4 mile grid across five national forests and the Columbia River Gorge National Scenic Area.
East of the Cascade Crest, published studies of air quality assessments using lichens are scarce. However, due to similarities in climate, vegetation, and lichen flora, those interested in Oregon and Washington air quality issues may find it useful to refer to some of the various baseline and point source studies in Idaho and Montana (Rope and Pearson 1990, Hoffman 1974, St. Clair and Newberry 1995), Wyoming (Gough and Erdman 1977) and Colorado (Jackson et al. 1996, Hale 1981).

